Chemistry, 30.03.2020 20:39, lexhorton2002
Liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 3.4 g of hexane is mixed with 22.6 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1
Do you know the correct answer?
Liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . S...
Questions in other subjects:





Chemistry, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00



Arts, 21.01.2022 14:00


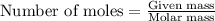 .....(1)
.....(1)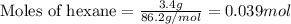
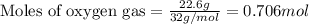
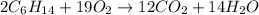
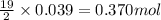 of oxygen gas
of oxygen gas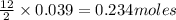 of carbon dioxide
of carbon dioxide