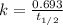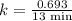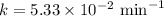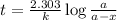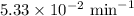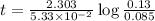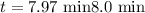Chemistry, 30.03.2020 20:27, smancilla2020
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. A) 12 B) 10. C) 8.0 D) 11 E) 5.0 C

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1
Do you know the correct answer?
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13...
Questions in other subjects:

History, 30.08.2019 08:30



Mathematics, 30.08.2019 08:30

Mathematics, 30.08.2019 08:30


Chemistry, 30.08.2019 08:30

Social Studies, 30.08.2019 08:30

Biology, 30.08.2019 08:30