
Chemistry, 30.03.2020 20:24, jonmorton159
Consider the following reaction at 298 K. C ( graphite ) + 2 H 2 ( g ) ⟶ CH 4 ( g ) Δ H ∘ = − 74.6 kJ and Δ S ∘ = − 80.8 J / K Calculate the following quantities. Δ S sys = J/K Δ S surr = J/K Δ S univ = J/K Is this reaction spontaneous? yes no

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 23:10, ArielA13
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
Do you know the correct answer?
Consider the following reaction at 298 K. C ( graphite ) + 2 H 2 ( g ) ⟶ CH 4 ( g ) Δ H ∘ = − 74.6 k...
Questions in other subjects:


Mathematics, 29.01.2020 08:04




Mathematics, 29.01.2020 08:04

Chemistry, 29.01.2020 08:04

Biology, 29.01.2020 08:04

Business, 29.01.2020 08:04


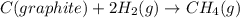
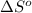 = Entropy of system = -80.8 J/K
= Entropy of system = -80.8 J/K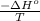
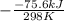
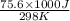
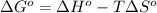
 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?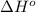 = standard enthalpy = -74600 J
= standard enthalpy = -74600 J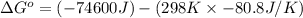
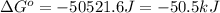
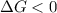 A reaction to be non-spontaneous when
A reaction to be non-spontaneous when 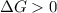
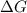 is negative or we can say that the value of
is negative or we can say that the value of 



