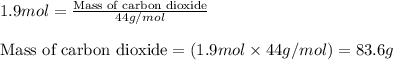Chemistry, 30.03.2020 22:16, janahiac09
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of carbon were burned in the presence of 73.8 g of oxygen, 13.0 g of oxygen remained unreacted. What mass of carbon dioxide was produced? Express your answer to one decimal place and include the appropriate units. View Available Hint(s)

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
Do you know the correct answer?
Part B When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 22.8 g of ca...
Questions in other subjects:



Mathematics, 07.05.2020 05:03

Mathematics, 07.05.2020 05:03



Mathematics, 07.05.2020 05:03

English, 07.05.2020 05:03


Mathematics, 07.05.2020 05:03


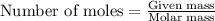 .....(1)
.....(1)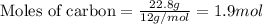
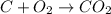
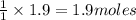 of carbon dioxide gas
of carbon dioxide gas