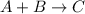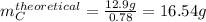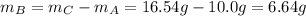Chemistry, 30.03.2020 20:11, sydneyfarrimonp9k3j4
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual percent yield of C in this reaction is 78%. Suppose 10.0 g of A are reacted with excess Compound B, and 12.9g of Compound C are successfully isolated at the end of the reaction.
1. What was the theoretical yield of C?
2. How much B was consumed by the reaction?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
Do you know the correct answer?
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual per...
Questions in other subjects:

Biology, 10.06.2020 07:57

Mathematics, 10.06.2020 07:57

History, 10.06.2020 07:57

Mathematics, 10.06.2020 07:57


English, 10.06.2020 07:57

Social Studies, 10.06.2020 07:57


Mathematics, 10.06.2020 07:57

Chemistry, 10.06.2020 07:57