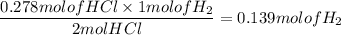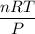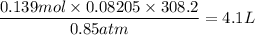Chemistry, 30.03.2020 19:29, dazesreplayy5363
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved. Calculate the volume(in L) of H2(g) at 35.2°C and 646 torr that can be formed when 325 mL of 0.855 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
Mg(s) + HCl(aq) --> MgCl2(aq) + H2(g) (unbalanced)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
Do you know the correct answer?
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved. Calcula...
Questions in other subjects:

Mathematics, 13.07.2019 15:00


Mathematics, 13.07.2019 15:00

Mathematics, 13.07.2019 15:00

Mathematics, 13.07.2019 15:00

History, 13.07.2019 15:00

Mathematics, 13.07.2019 15:00

History, 13.07.2019 15:00

Mathematics, 13.07.2019 15:00