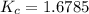Chemistry, 30.03.2020 19:34, jallison61
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially dissociates by the reaction below. 2 NH3(g) equilibrium reaction arrow N2(g) 3 H2(g) At equilibrium, 2.0 mol NH3 remains. What is the value of K for this reaction

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3



Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1
Do you know the correct answer?
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially di...
Questions in other subjects:


Biology, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00



Mathematics, 07.07.2019 16:00




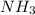 = 4.0 mole
= 4.0 mole
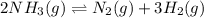
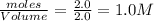
![K_c=\frac{[x]\times [3x]^3}{[(2-2x)]^2}](/tpl/images/0570/9112/70e97.png)
![K_c=\frac{[0.5]\times [3\times 0.5]^3}{[(2-2\times 0.5)]^2}](/tpl/images/0570/9112/97489.png)