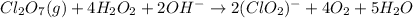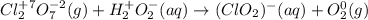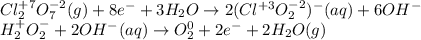Chemistry, 30.03.2020 19:38, ashley4329
Identify the oxidizing and reducing agents in the skeletal (unbalanced) reaction. Then, balance the reaction, including the phase (solid, liquid, etc.) of each species. The reaction takes place in basic aqueous solution. Cl 2 O 7 ( g ) + H 2 O 2 ( aq ) ⟶ ClO − 2 ( aq ) + O 2 ( g )

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
Do you know the correct answer?
Identify the oxidizing and reducing agents in the skeletal (unbalanced) reaction. Then, balance the...
Questions in other subjects: