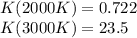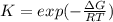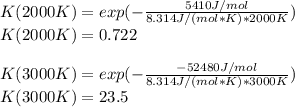The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X 2 ( g ) ⟶ X ( g ) Assume that the standard molar Gibbs energy of formation of X(g) is 5.41 kJ⋅mol − 1 at 2000. K and − 52.48 kJ⋅mol − 1 at 3000. K. Determine the value of K (the thermodynamic equilibrium constant) at each temperature. K at 2000. K = K at 3000. K =

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1


Do you know the correct answer?
The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X...
Questions in other subjects:





Mathematics, 03.12.2020 17:10

Social Studies, 03.12.2020 17:10

English, 03.12.2020 17:10



Spanish, 03.12.2020 17:10