
Chemistry, 30.03.2020 19:25, hsjsjsjdjjd
When a 3.80 g sample of C8H18(l) is burned in a bomb calorimeter, the temperature of the calorimeter rises by 27.3 oC. The heat capacity of the calorimeter, measured in a separate experiment, is 6.18 kJ/oC. Determine the ΔE for C8H18(l) in units of kJ/ mole C8H18(l).

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
Do you know the correct answer?
When a 3.80 g sample of C8H18(l) is burned in a bomb calorimeter, the temperature of the calorimeter...
Questions in other subjects:



Biology, 19.06.2020 14:57

Business, 19.06.2020 14:57



Mathematics, 19.06.2020 14:57

Mathematics, 19.06.2020 14:57




 = change in temperature = 27.3°C
= change in temperature = 27.3°C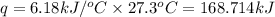


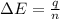
 = enthalpy change of the reaction
= enthalpy change of the reaction




