

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2
Do you know the correct answer?
A solution containing CaCl2 is mixed with a solution of Li2C2O4 to form a solution that is 3.5 × 10-...
Questions in other subjects:

Computers and Technology, 23.07.2019 10:00

Biology, 23.07.2019 10:00





Arts, 23.07.2019 10:00

Social Studies, 23.07.2019 10:00

Chemistry, 23.07.2019 10:00

Social Studies, 23.07.2019 10:00


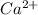 =
= 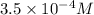
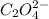 =
= 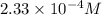
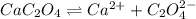
![K_{sp}=[Ca^{2+}][C_2O_4^{2-}]=2.3\times 10^{-9}](/tpl/images/0570/7450/df299.png)
![Q_{sp}=[Ca^{2+}][C_2O_4^{2-}]](/tpl/images/0570/7450/af00c.png)
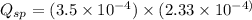
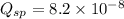
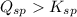 that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.
that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.



