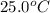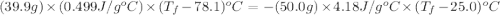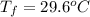A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initially at 25.0 °C and allowed to reach thermal equilibrium. What is the final temperature of the iron and water, given that the specific heat of iron is 0.449 J/(g⋅°C)? Assume no heat is lost to surroundings.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2


Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Do you know the correct answer?
A hot lump of 39.9 g of iron at an initial temperature of 78.1 °C is placed in 50.0 mL H 2 O initial...
Questions in other subjects:


Mathematics, 09.10.2020 21:01

Social Studies, 09.10.2020 21:01

Mathematics, 09.10.2020 21:01






Mathematics, 09.10.2020 21:01


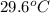
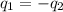
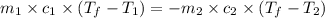
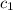 = specific heat of iron =
= specific heat of iron = 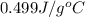
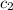 = specific heat of water =
= specific heat of water = 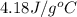
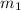 = mass of iron = 39.9 g
= mass of iron = 39.9 g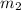 = mass of water =
= mass of water = 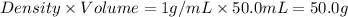
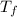 = final temperature of mixture = ?
= final temperature of mixture = ?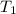 = initial temperature of iron =
= initial temperature of iron = 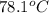
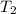 = initial temperature of water =
= initial temperature of water =