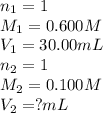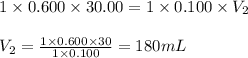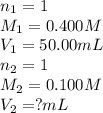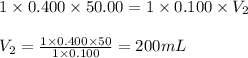Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
Do you know the correct answer?
Determine the volume at the equivalence point if a 0.100 M NaOH(aq) solution is used to titrate the...
Questions in other subjects:

Biology, 06.03.2021 02:20

Mathematics, 06.03.2021 02:20

History, 06.03.2021 02:20

Mathematics, 06.03.2021 02:20


Mathematics, 06.03.2021 02:20

Mathematics, 06.03.2021 02:20

Chemistry, 06.03.2021 02:20



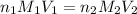
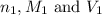 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid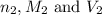 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base