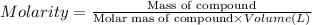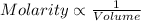Your lab partner told you that he measured out 25.0 mL of the unknown acid solution. But he actually went above the line on the graduated cylinder (added more than 25.0 mL). Would your final calculated molarity of the unknown acid be higher, lower or equal to the actual concentration

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
Do you know the correct answer?
Your lab partner told you that he measured out 25.0 mL of the unknown acid solution. But he actually...
Questions in other subjects:

Mathematics, 27.01.2021 19:30

Mathematics, 27.01.2021 19:30

Mathematics, 27.01.2021 19:30

Mathematics, 27.01.2021 19:30

Social Studies, 27.01.2021 19:30

English, 27.01.2021 19:30


Mathematics, 27.01.2021 19:30


English, 27.01.2021 19:30