

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2


Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 06:40, donalyndearingbizz
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
Do you know the correct answer?
Suppose in an experiment to determine the amount of sodium hypochlorite in bleach, 0.0000524 mol K I...
Questions in other subjects:


Mathematics, 04.09.2019 01:20

Chemistry, 04.09.2019 01:20


Mathematics, 04.09.2019 01:20


Mathematics, 04.09.2019 01:20

Mathematics, 04.09.2019 01:20

Mathematics, 04.09.2019 01:20

Mathematics, 04.09.2019 01:20


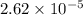 moles
moles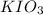 solution given = 0.0000524 moles
solution given = 0.0000524 moles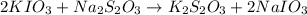
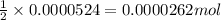 of sodium thiosulfate
of sodium thiosulfate



