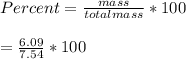Chemistry, 30.03.2020 17:42, haileesprague3999
Chalk is a mixture of CaCO3 and CaSO4. When added to HCl, only the CaCO3 reacts according to the reaction:
CaCO3(s) + 2 HCl (aq) → CaCl2 (aq) + CO2(g) + H2O (l)
When a 7.54 g piece of chalk is placed in HCl, 2.68 of CO2 is produced. What percentage of the chalk is CaCO3?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1



Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
Do you know the correct answer?
Chalk is a mixture of CaCO3 and CaSO4. When added to HCl, only the CaCO3 reacts according to the rea...
Questions in other subjects:





Biology, 21.06.2021 20:20


Mathematics, 21.06.2021 20:20

History, 21.06.2021 20:20


Mathematics, 21.06.2021 20:20