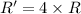Chemistry, 30.03.2020 17:31, angelolucero146
) Given the following rate law, how does the rate of reaction change if the concentration of Y is doubled? Rate = k [X][Y]2 A) The rate of reaction will increase by a factor of 2. B) The rate of reaction will increase by a factor of 5. C) The rate of reaction will increase by a factor of 4. D) The rate of reaction will decrease by a factor of 2. E) The rate of reaction will remain unchanged.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
Do you know the correct answer?
) Given the following rate law, how does the rate of reaction change if the concentration of Y is do...
Questions in other subjects:




Mathematics, 12.01.2021 06:10

Mathematics, 12.01.2021 06:10


Computers and Technology, 12.01.2021 06:10


Chemistry, 12.01.2021 06:10

Mathematics, 12.01.2021 06:10


![Rate(R) = k [X][Y]^2](/tpl/images/0570/5331/25584.png)
![R'=k[X][2Y]^2](/tpl/images/0570/5331/8ce13.png)
![R'=k[X]4[Y]^2](/tpl/images/0570/5331/f56ad.png)
![R'=4k[X][Y]^2](/tpl/images/0570/5331/59783.png)