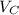Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
Do you know the correct answer?
In a liquid mixture of chloroform (C) and acetone (A) with chloroform mole fraction xC = 0.4693, the...
Questions in other subjects:

Mathematics, 21.07.2021 18:00

Mathematics, 21.07.2021 18:00

Chemistry, 21.07.2021 18:00


Computers and Technology, 21.07.2021 18:00

Mathematics, 21.07.2021 18:00

Mathematics, 21.07.2021 18:00



Mathematics, 21.07.2021 18:10


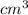
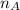
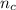
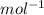
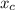 ) × 58.08 + n
) × 58.08 + n 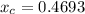
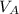 +
+