
Chemistry, 30.03.2020 16:55, jc95704816
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assume that all the radiation is absorbed and converted to heat. How many photons are required for the liquid to absorb 58.28 J 58.28 J of heat?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
Do you know the correct answer?
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assum...
Questions in other subjects:


Mathematics, 05.05.2020 14:06


History, 05.05.2020 14:06

English, 05.05.2020 14:06

English, 05.05.2020 14:06





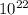 Photons
Photons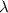 = 6.92 ×
= 6.92 ×  cm = 6.92 ×
cm = 6.92 × 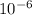 m
m
 J - s
J - s

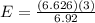 ×
× 

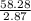 ×
× 



