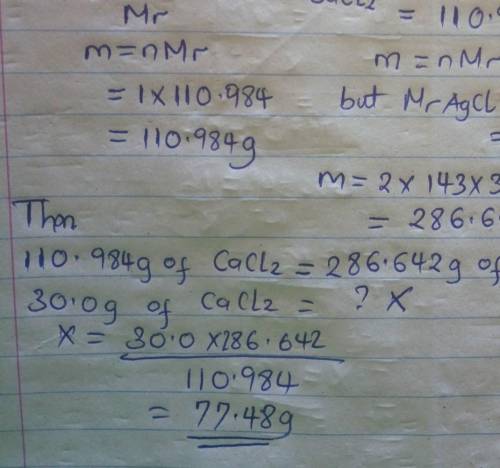The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgN...

Chemistry, 30.03.2020 17:04, simoncastro1
The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl + Ca(NO3)2
How many grams of AgCl are produced from 30.0 grams of CaCl2?
(Molar mass of Ca = 40.078 g/mol, Cl = 35.453 g/mol, O = 15.999 g/mol, Ag = 107.868 g/mol, N = 14.007 g/mol) (1 point)
19.4 grams
38.8 grams
58.2 grams
77.5 grams

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 30.09.2019 20:30




Social Studies, 30.09.2019 20:30


Social Studies, 30.09.2019 20:30