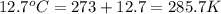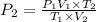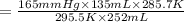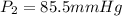Chemistry, 30.03.2020 16:52, haelleydorethy
Two gas-containers, A and B, are connected with a valve. The first container, A, has a volume of 135 mL, and the second container, B, has a volume of 117 mL. A sample of gas originally in container A is at 22.5 C and the pressure is 165 mmHg. What is the pressure (in mmHg) of the gas sample when the valve is opened and the gas now occupies both containers at a temperature of 12.7 C

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Averybeam300
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1


Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Two gas-containers, A and B, are connected with a valve. The first container, A, has a volume of 135...
Questions in other subjects:

Mathematics, 06.10.2020 14:01

English, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01


English, 06.10.2020 14:01



Chemistry, 06.10.2020 14:01

History, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01


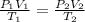
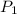 = initial pressure of gas in container A = 165 mmHg
= initial pressure of gas in container A = 165 mmHg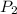 = final pressure of gas = ?
= final pressure of gas = ?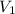 = initial volume of gas in container A=
= initial volume of gas in container A= 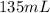
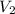 = final volume of gas = 135 mL + 117 mL = 252 mL
= final volume of gas = 135 mL + 117 mL = 252 mL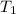 = initial temperature of gas in container A =
= initial temperature of gas in container A = 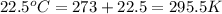
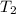 = final temperature of gas =
= final temperature of gas =