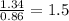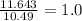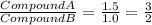Chemistry, 30.03.2020 16:16, kenishawilkinsoy4mgw
Sulfur reacts with oxygen and creates two compounds. Compound A contains 1.34 g of sulfur for every 0.86 g of oxygen. Compound B contains 11.63 g of sulfur for every 10.49 g of oxygen. What is the mass ratio of oxygen rounded to the nearest whole number

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
Do you know the correct answer?
Sulfur reacts with oxygen and creates two compounds. Compound A contains 1.34 g of sulfur for every...
Questions in other subjects:



Biology, 25.11.2021 06:40



Mathematics, 25.11.2021 06:40




Mathematics, 25.11.2021 06:40