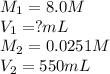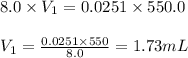A chemist must prepare 550 mL of hydrochloric acid solution with a pH of 1.60 at 25°C .
He wi...

A chemist must prepare 550 mL of hydrochloric acid solution with a pH of 1.60 at 25°C .
He will do this in three steps:
-Fill a 550.0 mL volumetric flask about halfway with distilled water.
-Measure out a small volume of concentrated (8.0M) stock hydrochloric acid solution and add it to the flask.
-Fill the flask to the mark with distilled water.
Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2


Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Advanced Placement (AP), 10.02.2021 20:20




Mathematics, 10.02.2021 20:20


History, 10.02.2021 20:20


![pH=-\log[H^+]](/tpl/images/0570/3526/cf945.png)
![1.60=-\log [H^+]](/tpl/images/0570/3526/bdcbd.png)
![[H^+]=10^{-1.60}=0.0251M](/tpl/images/0570/3526/4bcd7.png)
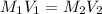
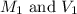 are the molarity and volume of the concentrated HCl solution
are the molarity and volume of the concentrated HCl solution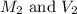 are the molarity and volume of diluted HCl solution
are the molarity and volume of diluted HCl solution