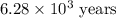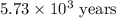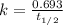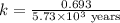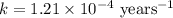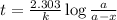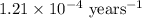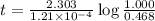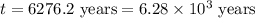Chemistry, 30.03.2020 15:41, SMURFETTE86
The half-life for the radioactive decay of carbon-14 to nitrogen-14 is 5.73 x 10^3 years. Suppose nuclear chemical analysis shows that there is 0.523mmol of nitrogen-14 for every 1.000 mmol of carbon-14 in a certain sample of rock.
Calculate the age of the rock. Round your answer to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, alejandra1201
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2


Chemistry, 23.06.2019 08:00, vetterk1400
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
Do you know the correct answer?
The half-life for the radioactive decay of carbon-14 to nitrogen-14 is 5.73 x 10^3 years. Suppose nu...
Questions in other subjects:



Geography, 08.09.2021 02:50

Mathematics, 08.09.2021 02:50


Mathematics, 08.09.2021 02:50



English, 08.09.2021 02:50

Social Studies, 08.09.2021 02:50