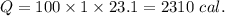Chemistry, 29.03.2020 22:37, Candieboo6939
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to rise from 24.0°C go 47.1 °C.
Calculate the number of calories absorbed by the WATER as the Ritz crackers burn. (Use the specific heat of water in terms of calories: 1.0 cal/ g °C) ** Remember you are calculating the heat gained by the water, NOT the heat lost by the Ritz cracker - use the correct mass!!
12.7 cal
53.2 cal
2310 cal
9.67 x 105 cal

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, juansantos7b
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1
Do you know the correct answer?
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to ris...
Questions in other subjects:



Mathematics, 22.04.2020 00:37

Social Studies, 22.04.2020 00:37


Mathematics, 22.04.2020 00:37




Mathematics, 22.04.2020 00:38