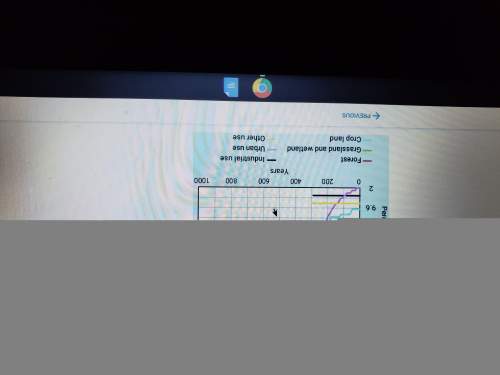
Chemistry, 29.03.2020 22:17, alligatorsocks
25.0 g of a substance requires 117.4 Joules of energy to raise its temperature from 75.0 to 87.2 oC. What is the specific heat of the substance?
.054 J/g°C
.063 J/g°C
2.60 J/g°C
.385 J/g°C

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
Do you know the correct answer?
25.0 g of a substance requires 117.4 Joules of energy to raise its temperature from 75.0 to 87.2 oC....
Questions in other subjects:




Mathematics, 09.01.2021 02:50

Biology, 09.01.2021 02:50

Mathematics, 09.01.2021 02:50

Mathematics, 09.01.2021 02:50


Mathematics, 09.01.2021 02:50


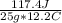 = 0.385 J/g°C
= 0.385 J/g°C