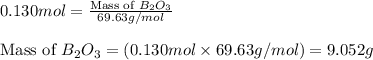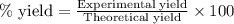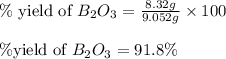Chemistry, 29.03.2020 21:45, demarpratt1270
Find percent yield:
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10.00 g of
O2 is 8.32 g. What is the percent yield?
2 B3H9 + 12 O2 => 5 B2O3 +9 H2O
87.2
92.8
91.8
75.5
74.5

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 01:30, montoyaricardo3550
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3
Do you know the correct answer?
Find percent yield:
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10....
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10....
Questions in other subjects:

Mathematics, 01.12.2019 05:31

English, 01.12.2019 05:31


Biology, 01.12.2019 05:31


Mathematics, 01.12.2019 05:31


Mathematics, 01.12.2019 05:31


Mathematics, 01.12.2019 05:31


 .....(1)
.....(1)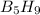 :
: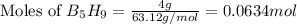
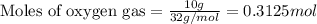
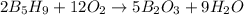
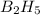
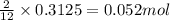 of
of 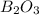
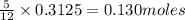 of water
of water