
How can an unknown A Heaction be determined using Hess's law?
A. The free energy of the reaction is used to determine the A H for
the reaction.
O
B. The reaction is repeated at different temperatures to determine
the A Hreaction
O
C. Enthalpies from reaction steps are added to determine an
unknown A Hreaction:
D. The unknown A Heaction is determined after the reaction is run in a
calorimeter.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, KindaSmartPersonn
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
Do you know the correct answer?
How can an unknown A Heaction be determined using Hess's law?
A. The free energy of the reacti...
A. The free energy of the reacti...
Questions in other subjects:


Chemistry, 04.09.2020 01:01

Mathematics, 04.09.2020 01:01



English, 04.09.2020 01:01

Mathematics, 04.09.2020 01:01




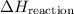 from reaction steps are combined to determine an unknown
from reaction steps are combined to determine an unknown 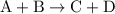 .
. 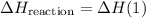 .Reaction (2):
.Reaction (2): 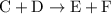 .
. 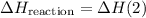 .
.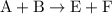 .
.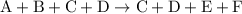 .
. and
and 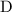 to get:
to get: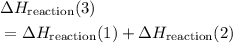 .
.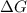 is involved in this calculation. There's not even the need to carry out an experiment or take any new measurements. Because of that, Hess's Law can be very useful for finding the
is involved in this calculation. There's not even the need to carry out an experiment or take any new measurements. Because of that, Hess's Law can be very useful for finding the 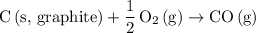 .
.



