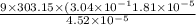Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 23.06.2019 14:00, cfonse11
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1

Chemistry, 23.06.2019 22:00, garciagang0630
Athermometer is removed from a room where the temperature is 70° f and is taken outside, where the air temperature is 30° f. after one-half minute the thermometer reads 50° f. what is the reading of the thermometer at t = 1 min? (round your answer to two decimal places.)
Answers: 3

Chemistry, 24.06.2019 10:00, MileenaKitana
Assume that the water stream is replaced by a stream of ccl4. predict what would happen in each case:
Answers: 1
Do you know the correct answer?
For water at 30C and 1 atm: a= 3.04x10^-4 k^-1 , k =4.52x10^-5 atm ^-1 = 4.46 x10^-10 m^2/N, cpm= 75...
Questions in other subjects:

Biology, 12.10.2020 21:01

Spanish, 12.10.2020 21:01



English, 12.10.2020 21:01



Computers and Technology, 12.10.2020 21:01



 of water at 30C and 1 atm is 256.834 J/mol·K.
of water at 30C and 1 atm is 256.834 J/mol·K.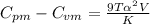
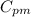 = Specific heat of gas at constant pressure = 75.3 J/mol·K
= Specific heat of gas at constant pressure = 75.3 J/mol·K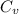 =
=