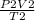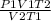Working with the Combined Gas Law
QUICK CHECK
PV, PT
Numerator (A):
O P VaTz
O PzV2T1
OP, V,
Using the combined gas law above, identify the
variables that would be in the numerator (A) and
denominator (B) if you were to rearrange the gas
law to solve for final pressure.
DONE
А
P2 =
Denominator (B):
OP, V,Т2
O PzV2T1
o TV2
OP, V,
ME
Intro

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3



Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
Do you know the correct answer?
Working with the Combined Gas Law
QUICK CHECK
PV, PT
Numerator (A):
O P VaTz...
QUICK CHECK
PV, PT
Numerator (A):
O P VaTz...
Questions in other subjects:

Chemistry, 16.07.2019 06:30

Chemistry, 16.07.2019 06:30

Biology, 16.07.2019 06:30

History, 16.07.2019 06:30



Mathematics, 16.07.2019 06:30




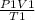 =
=