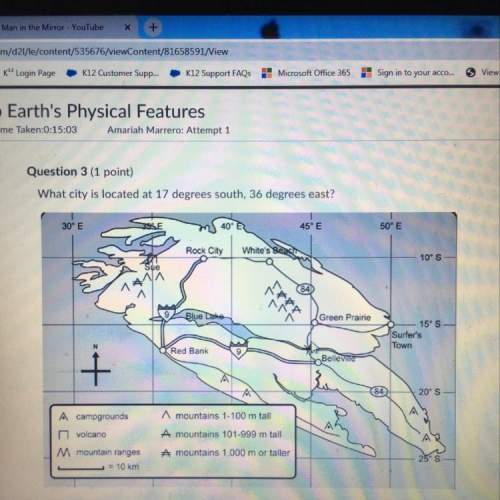
The volume of a given mass of gas varies inversely with pressure, provided that the
temperature remains constant because
1. the average Kinetic energy of the molecules of a gas is proportional to the absolute temperature.
2.attractive forces between gas molecules are appreciable.
3.collisions between gas molecules are perfectly elastic.
4.Increasing the molecular concentration at constant temperature means increasing the number of collisions between molecules and container
5.attractive forces between a molecule are negligible.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
Do you know the correct answer?
The volume of a given mass of gas varies inversely with pressure, provided that the
temperatur...
temperatur...
Questions in other subjects:

Social Studies, 26.08.2021 23:10


Business, 26.08.2021 23:10



Mathematics, 26.08.2021 23:10


Social Studies, 26.08.2021 23:10

Mathematics, 26.08.2021 23:10