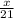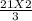Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
...

Chemistry, 28.03.2020 22:17, NetherisIsTheQueen
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
3H2(g)+N2(g)→2NH3(g)
How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Do you know the correct answer?
Questions in other subjects:


SAT, 04.07.2019 06:00



Mathematics, 04.07.2019 06:00

Social Studies, 04.07.2019 06:00



Chemistry, 04.07.2019 06:00

Mathematics, 04.07.2019 06:00


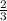 =
=