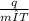A 63.5 g sample of an unidentified metal absorbs 355 ) of heat when its temperature changes
by...

Chemistry, 28.03.2020 21:36, iamastudent79
A 63.5 g sample of an unidentified metal absorbs 355 ) of heat when its temperature changes
by 4.56 °C. Calculate the specific heat capacity of the metal.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1



Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 04.05.2020 22:38

Mathematics, 04.05.2020 22:38


Spanish, 04.05.2020 22:38

Chemistry, 04.05.2020 22:38

English, 04.05.2020 22:38


Biology, 04.05.2020 22:38