
Chemistry, 28.03.2020 05:51, aricketts3901
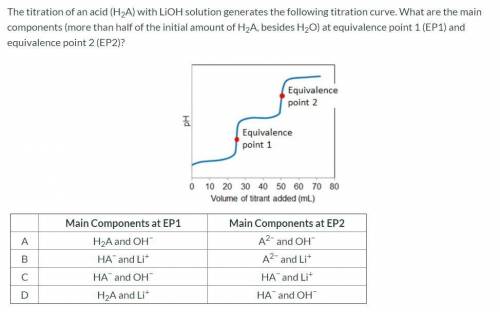
The titration of an acid (H2A) with LiOH solution generates the following titration curve. What are the main components (more than half of the initial amount of H2A, besides H2O) at equivalence point 1 (EP1) and equivalence point 2 (EP2)?
Main Components at EP1 Main Components at EP2
A H2A and OH− A2− and OH−
B HA− and Li+ A2− and Li+
C HA− and OH− HA− and Li+
D H2A and Li+ HA− and OH−

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:40, ohgeezy
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
Do you know the correct answer?
The titration of an acid (H2A) with LiOH solution generates the following titration curve. What are...
Questions in other subjects:


Mathematics, 06.04.2020 04:13

English, 06.04.2020 04:13




History, 06.04.2020 04:14


Mathematics, 06.04.2020 04:14






