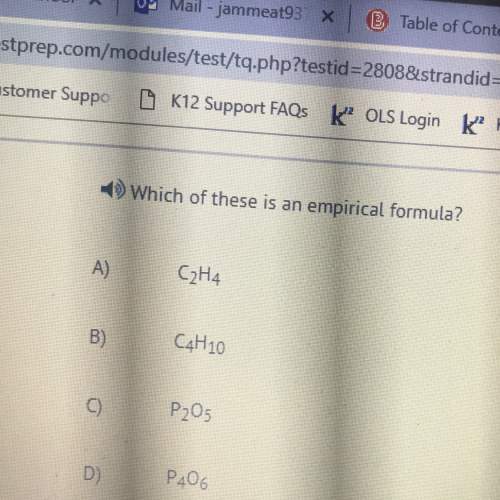
Chemistry, 28.03.2020 03:00, dathanboyd
The process in which an organic acid and an alcohol react to form an ester and water is known as esterification. Ethyl butanoate (C3H7COOC2H5) an ester is formed when the alcohol ethanol (C2H5OH) and butanoic acid (C3H7COOH) are heated in the presence of sulfuric acid.
C2H5OH(l) + C3H7COOH(l) C3H7COOC2H5(l) + H2O(l)
Determine the mass of ethyl butanoate produced if 5.90 mol of ethanol is used.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
Do you know the correct answer?
The process in which an organic acid and an alcohol react to form an ester and water is known as est...
Questions in other subjects:


Mathematics, 22.06.2019 17:40



Mathematics, 22.06.2019 17:40

Mathematics, 22.06.2019 17:40

Biology, 22.06.2019 17:40



Biology, 22.06.2019 17:40