
Chemistry, 28.03.2020 03:02, Derrick253
If the pressure P applied to a gas is increased while the gas is held at a constant temperature, then the volume V of the gas will decrease. The rate of change of the volume of gas with respect to the pressure is proportional to the reciprocal of the square of the pressure. Which of the following is a differential equation that could describe this relationship?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
Do you know the correct answer?
If the pressure P applied to a gas is increased while the gas is held at a constant temperature, the...
Questions in other subjects:

Physics, 19.11.2020 01:00


Mathematics, 19.11.2020 01:00

Arts, 19.11.2020 01:00


Mathematics, 19.11.2020 01:00



Biology, 19.11.2020 01:00


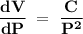 .
. Volume = Constant
Volume = Constant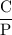
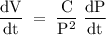
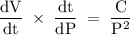
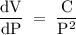
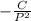 .
. 
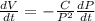 which gives
which gives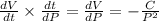
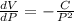 .
.



