Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:30, smartie80
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3
Do you know the correct answer?
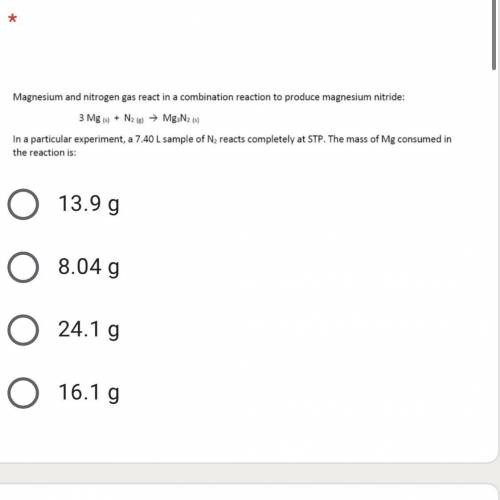
A 7.40 L sample of N2 reacts completely at STP. the mass of the Mg consumed in the reaction is:
Questions in other subjects:


Mathematics, 18.02.2022 18:20

Health, 18.02.2022 18:20






Physics, 18.02.2022 18:20