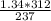Chemistry, 27.03.2020 22:28, LaughingAlanna
A gas has a pressure of 1.34 atm when the temperature is 237K. The gas is then heated until the temperature measures 312K. What will be the new pressure?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
Do you know the correct answer?
A gas has a pressure of 1.34 atm when the temperature is 237K. The gas is then heated until the temp...
Questions in other subjects:



History, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00


Mathematics, 13.05.2021 01:00


Mathematics, 13.05.2021 01:00


History, 13.05.2021 01:00


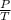 = constant
= constant 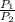 =
= 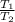
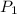 = initial pressure of a gas
= initial pressure of a gas 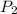 = final pressure of a gas
= final pressure of a gas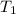 = initial temperature of a gas
= initial temperature of a gas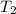 = final temperature of a gas
= final temperature of a gas