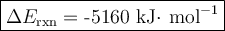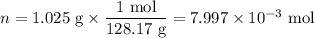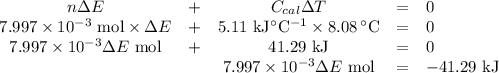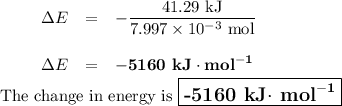Chemistry, 27.03.2020 21:01, usagimiller
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 gof naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘C to 32.33 ∘C. Find ΔErxn for the combustion of naphthalene. The heat capacity of the calorimeter, determined in a separate experiment, is 5.11kJ/∘C. Express the change in energy in kilojoules per mole to three significant figures.ΔErxn = kJ/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1
Do you know the correct answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 gof naphthalene...
Questions in other subjects:

Physics, 07.03.2021 14:00

Mathematics, 07.03.2021 14:00

Spanish, 07.03.2021 14:00

Chemistry, 07.03.2021 14:00

Mathematics, 07.03.2021 14:00



Chemistry, 07.03.2021 14:00

Mathematics, 07.03.2021 14:00