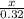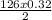Chemistry, 27.03.2020 20:59, kyrabrown33
How many kJ of heat are released by the reaction of 25.0 g of Na2O2(s) in the following reaction? (M = 78.0 g/mol for Na2O2)
2 Na2O2(s) + 2 H2O(l) → 4 NaOH(aq) + O2(g) ∆Hο = −126 kJ

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1


Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
Do you know the correct answer?
How many kJ of heat are released by the reaction of 25.0 g of Na2O2(s) in the following reaction? (M...
Questions in other subjects:



Mathematics, 04.12.2019 04:31


Biology, 04.12.2019 04:31

Mathematics, 04.12.2019 04:31

Chemistry, 04.12.2019 04:31




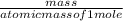
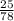
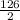 =
=