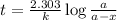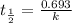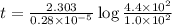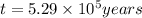Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If there are 4.4 x 102 g of the isotope in a small atomic bomb, how long will it take (in yr) for the substance to decay to 1.0 x 102 g, too small an amount for an effective bomb? This radioactive decay follows first order kinetics.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
Do you know the correct answer?
Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If ther...
Questions in other subjects:

English, 04.12.2020 20:20

Mathematics, 04.12.2020 20:20


Mathematics, 04.12.2020 20:20

Mathematics, 04.12.2020 20:20


Mathematics, 04.12.2020 20:20


Computers and Technology, 04.12.2020 20:20

Mathematics, 04.12.2020 20:20


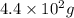 to
to  is
is