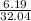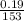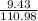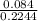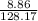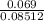Chemistry, 27.03.2020 19:31, carlosleblanc26
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methanol (CH3OH) in 1.50 × 102 mL of solution:
mol/L
(b) 9.43 g of calcium chloride (CaCl2) in 2.20 × 102 mL of solution:
mol/L
(c) 8.86 g of naphthalene (C10H8) in 85.2 mL of benzene solution:
mol/L

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
Do you know the correct answer?
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methano...
(a) 6.19 g of methano...
Questions in other subjects:


History, 29.01.2020 02:11

Mathematics, 29.01.2020 02:11

Biology, 29.01.2020 02:11

Social Studies, 29.01.2020 02:11

Mathematics, 29.01.2020 02:11

Chemistry, 29.01.2020 02:11

Chemistry, 29.01.2020 02:11


History, 29.01.2020 02:11


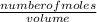 equation 1
equation 1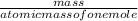 equation 2
equation 2