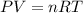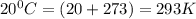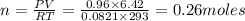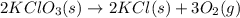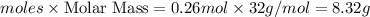Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
<...

Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2KClO3(s) → 2KCl(s) + 3O2(g)
The product gas, O2, is collected over water at a temperature of 20 °C and a pressure of 747 mm Hg. If the wet O2 gas formed occupies a volume of 6.42 L, the number of grams of O2 formed is g. The vapor pressure of water is 17.5 mm Hg at 20 °C.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 10:30, aliyyahlove
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1

Chemistry, 23.06.2019 15:30, dpazmembreno
How is the electron sea model of metallic bonding different from the band theory? how are they the same? give at least one similarity and one difference between the models
Answers: 3

Chemistry, 23.06.2019 15:50, tatiaa2155
Many radioactive atoms that have large masses undergo radioactive decay by releasing a particle that is identical to a helium-4 nucleus. what changes in the original atom are expected as a result of this natural phenomenon? the atomic number and the mass number will decrease. the atomic number and the mass number will increase. the atomic number will increase, and the mass number will decrease. the atomic number will decrease, and the mass number will increase.
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Biology, 22.06.2019 14:50

Mathematics, 22.06.2019 14:50

Mathematics, 22.06.2019 14:50

History, 22.06.2019 14:50


History, 22.06.2019 14:50

Biology, 22.06.2019 14:50

Mathematics, 22.06.2019 14:50

Mathematics, 22.06.2019 14:50


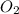 formed is 8.32 g
formed is 8.32 g