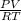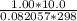Chemistry, 27.03.2020 18:26, ebookhardt917
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm. The volume of the helium is reduced to 10.4L, but the temperature and pressure of the gas are kept con- stant. What is the new quantity of the gas, in moles? Show all work using units and sig figs.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
Do you know the correct answer?
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm...
Questions in other subjects:



Mathematics, 21.06.2021 01:00




Mathematics, 21.06.2021 01:00


Mathematics, 21.06.2021 01:00

History, 21.06.2021 01:00