
Chemistry, 27.03.2020 16:45, janahiac09
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?
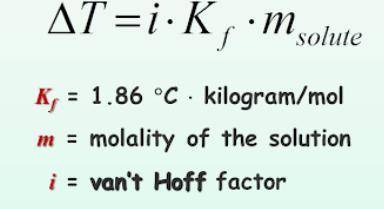

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1


Do you know the correct answer?
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?<...
Questions in other subjects:



Chemistry, 01.04.2020 17:26

Mathematics, 01.04.2020 17:26


Biology, 01.04.2020 17:26


Mathematics, 01.04.2020 17:26

World Languages, 01.04.2020 17:26

Mathematics, 01.04.2020 17:26






