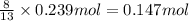Problem PageQuestion Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 4.6 g of butane is mixed with 7.65 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Do you know the correct answer?
Problem PageQuestion Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide...
Questions in other subjects:

Business, 16.12.2020 19:10

Mathematics, 16.12.2020 19:10

Mathematics, 16.12.2020 19:10

History, 16.12.2020 19:10

Mathematics, 16.12.2020 19:10


Mathematics, 16.12.2020 19:10

English, 16.12.2020 19:10



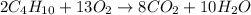
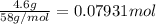
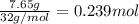
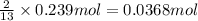 of butane.
of butane.