
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution at 22°C. Which statement explains why the contents of the flask are at equilibrium?The rate of dissolving is equal to the rate of crystallization. The rate of dissolving is greater than the rate of crystallization. The concentration of the solid is equal to the concentration of the solution. The concentration of the solid is greater than the concentration of the solution.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
Do you know the correct answer?
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution...
Questions in other subjects:


History, 18.08.2020 02:01

Mathematics, 18.08.2020 02:01




Mathematics, 18.08.2020 02:01



Social Studies, 18.08.2020 02:01


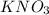 from aqueous
from aqueous 



