
Chemistry, 26.03.2020 22:48, giraffesaur44
Determine the expression for the rate of the reaction with respect to each of the reactants and products. Determine the expression for the rate of the reaction with respect to each of the reactants and products. Rate=−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]ΔtRate =−13Δ[A]Δt=−Δ[B]Δt=12Δ[C]Δt Rate=−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]ΔtRate =−12Δ[A]Δt=−Δ[B]Δt=13Δ[C]Δt Rate=−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]ΔtRate =−Δ[A]Δt=−12Δ[B]Δt=13Δ[C]Δt Rate=12Δ[A]Δt=12Δ[B]Δt=13Δ[C]Δt

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, Lesquirrel
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1
Do you know the correct answer?
Determine the expression for the rate of the reaction with respect to each of the reactants and prod...
Questions in other subjects:

Mathematics, 28.07.2019 16:00




Social Studies, 28.07.2019 16:00






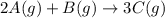
![Rate=-\frac{1}{3}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{2}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/ff0f8.png)
![Rate=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}=-\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/11b91.png)
![Rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/f7a0b.png)
![Rate=\frac{1}{2}\frac{\Delta [A]}{\Delta t}=\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/3152a.png)
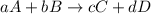
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0566/1120/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0566/1120/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0566/1120/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0566/1120/d4b94.png)
![\text{Rate of disappearance of }A=-\frac{1}{2}\frac{\Delta [A]}{\Delta t}](/tpl/images/0566/1120/4cd85.png)
![\text{Rate of disappearance of }B=-\frac{\Delta [B]}{\Delta t}](/tpl/images/0566/1120/43240.png)
![\text{Rate of formation of }C=+\frac{1}{3}\frac{\Delta [C]}{\Delta t}](/tpl/images/0566/1120/d1447.png)




