
Chemistry, 26.03.2020 22:42, elijahsantiago21
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 mL of a CH3COOH (acetic acid)/ CH3COONa * 3H2O buffer. The target pH of the buffer is 5.25. The given concentration of [CH3COOH] is equal to 0.10 M. Ka = 1.80 x 10-5 for acetic acid.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
Do you know the correct answer?
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 m...
Questions in other subjects:

History, 20.11.2020 22:40


Social Studies, 20.11.2020 22:40

Mathematics, 20.11.2020 22:40

English, 20.11.2020 22:40

English, 20.11.2020 22:40



Spanish, 20.11.2020 22:40


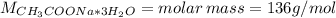
![pH = pKa + log(\frac{[CH_{3}COONa*3H_{2}O]}{[CH_{3}COOH]})](/tpl/images/0566/0820/ec252.png) (1)
(1)![log [CH_{3}COONa*3H_{2}O] = pH - pKa + log [CH_{3}COOH]](/tpl/images/0566/0820/d2774.png)
![log [CH_{3}COONa*3H_{2}O] = 5.25 - (-log(1.80 \cdot 10^{-5})) + log (0.10) = -0.495](/tpl/images/0566/0820/9589c.png)
![[CH_{3}COONa*3H_{2}O] = 10^{-0.495} = 0.32 M](/tpl/images/0566/0820/c6ecf.png)
![m = moles*M = [CH_{3}COONa*3H_{2}O]*V*M = 0.32 mol/L*0.250 L*136 g/mol = 10.88 g](/tpl/images/0566/0820/427f5.png)




