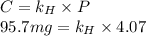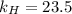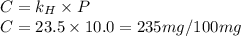Chemistry, 26.03.2020 22:40, xxgissellexx
At a certain temperature, the solubility of N2 gas in water at 4.07 atm is 95.7 mg of N2 gas/100 g water . Calculate the solubility of N2 gas in water, at the same temperature, if the partial pressure of N2 gas over the solution is increased from 4.07 atm to 10.0 atm .

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 23.06.2019 05:30, khaylaperry
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
Do you know the correct answer?
At a certain temperature, the solubility of N2 gas in water at 4.07 atm is 95.7 mg of N2 gas/100 g w...
Questions in other subjects:


Biology, 16.04.2021 19:50






Biology, 16.04.2021 19:50



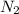 gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.
gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.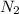 in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.
in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.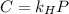 .
.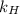 = Henry’s law constant = ?
= Henry’s law constant = ?